The reactions of UV precursors for gas barrier coatings are investigated using quantum chemical methods. The focus is on the identification of reaction pathways to reactants that adversely affect the coating properties. In addition, the properties of photoinitiators and molecular sensors are characterized using quantum chemical methods.
Expertise
- Characterization of photoinitiators and molecular sensors using quantum chemical methods
- Quantum chemical studies on the reactivity of molecular precursors for gas barrier coatings
Highlights
Low-Temperature Photochemical Conversion of Organometallic Precursor Layers to Titanium(IV) Oxide Thin Films
P. C. With, U. Helmstedt, S. Naumov, A. Sobottka, A. Prager, U. Decker, R. Heller, B. Abel, L. Prager
Chem. Mater. 28 (2016) 7715-7724
10.1021/acs.chemmater.6b02757Titanium oxide coatings can be obtained from organometallic precursors at atmospheric pressure and close to room temperature. Quantum chemical calculations complement the experimental work and provide insight into the mode of action.
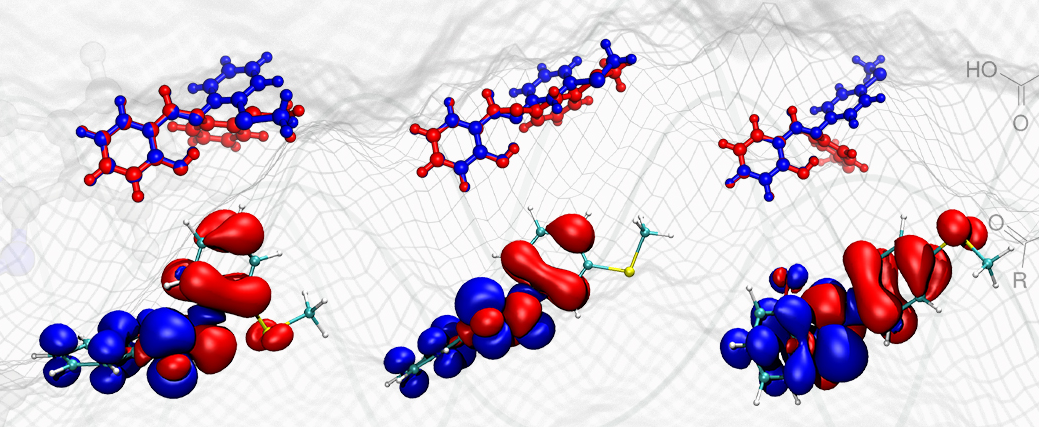
Peculiarities of the photoinitiator-free photopolymerization of pentabrominated and pentafluorinated aromatic acrylates and methacrylates
O. Daikos, S. Naumov, W. Knolle, K. Heymann, T. Scherzer
Phys. Chem. Chem. Phys. 18 (2016) 32369-32377
https://doi.org/10.1039/C6CP06549JThe influence of the halogen substituents on the photoinitiator-free polymerization of acrylates is investigated by experimental as well as quantum chemical approaches. Brominated, aromatic substituents result in a localization of the LUMO and the spin density on this functional group supporting a homolytic cleavage of the C-Br bond.
Light controlled oxidation by supramolecular Zn(II) Schiff-base complexes
C. Laube, J. A. Taut, J. Kretzschmar, S. Zahn, W. Knolle, S. Ullman, A. Kahnt, B. Kersting, B. Abel
Inorg. Chem. Front. 7 (2020) 4333-4346
https://doi.org/10.1039/D0QI00980FThe photochemical and reactive properties of a molecular sensor based on a Zn(II) salicylaldiminato-functionalized calixarene complex are characterized by experimental and computational approaches. The influence of a methoxy group on two homologous ligand systems is discussed in detail.