Molecular ions exhibit a variety of technologically relevant properties. The preparation of thin ionic surface layers is usually performed by deposition from salt solutions. We explore an alternative preparation method by depositing molecular ions from the gas phase. For this purpose, ions are transferred into a vacuum using electrospray. A desired ion is selected using mass spectrometric methods and subsequently gently deposited on a surface while maintaining the molecular integrity. One of our main goals is the preparation of functional surfaces with submonolayer coverages of redox-switchable complex ions for applications in molecular electronics. Furthermore, multilayer deposits of organometallic cations will be generated for electron beam-induced structuring processes, which could serve as novel nanoscale humidity sensors with high sensitivity.
Research within the joint lab “molecular ion soft-landing” is performed in direct cooperation with the junior research group of Dr. J. Warneke of Leipzig University.
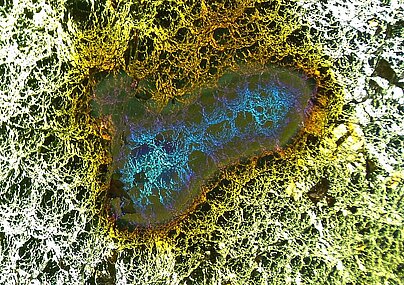
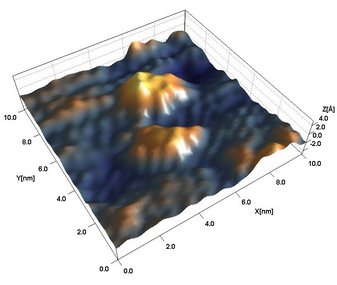
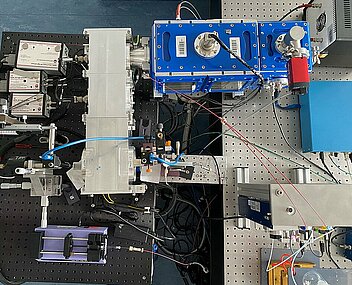
Expertise
- Soft-Landing of mass-selected ions with precisely controlled composition, charge state and kinetic energy
- Electron-induced chemistry on surfaces
- ESI mass spectrometry
Highlights
Anion-Anion Chemistry with Mass-Selected Molecular Fragments on Surfaces
F. Yang, K. A. Behrend, H. Knorke, M. Rohdenburg, A. Charvat, C. Jenne, B. Abel, J. Warneke
Angewandte Chemie Communication, First published: 14 September 2021
https://doi.org/10.1002/anie.202109249While reactions between ions and neutral molecules in the gas phase have been studied extensively, reactions between molecular ions of same polarity remain relatively unexplored. Herein we show that reactions between fragment ions generated in the gas phase and molecular ions of the same polarity are possible by soft-landing of both reagents on surfaces. The reactive [B12I11]1− anion was deposited on a surface layer built up by landing the generally unreactive [B12I12]2−. Ex-situ analysis of the generated material shows that [B24I23]3− was formed. A computational study shows that the product is metastable in the gas phase, but a charge balanced environment of a grounded surface may stabilize the triply charged product, as suggested by model calculations. This opens new opportunities for the generation of highly charged clusters using unconventional building blocks from the gas phase.
Insights from Adsorption and Electron Modification Studies of Polyoxometalates on Surface for Molecular Memory Applications
M. Moors, J. Warneke, X. López, C. de Graaf, B. Abel, K. Y. Monakhov
Acc. Chem. Res. (2021) 54, 17, 3377–3389
https://doi.org/10.1021/acs.accounts.1c00311This Account highlights recent experimental and theoretical work focusing on the development of polyoxometalates (POMs) as possible active switching units in what may be called “molecule-based memory cells”. Herein, we critically discuss how multiply charged vanadium-containing POMs, which exhibit stable metal–oxo bonds and are characterized by the excellent ability to change their redox states without significant structural distortions of the central polyoxoanion core, can be immobilized best and how they may work optimally at appropriate surfaces. Furthermore, we critically discuss important issues and challenges on the long way toward POM-based nanoelectronics.
On-Surface Single-Molecule Identification of Mass-Selected Cyclodextrin-Supported Polyoxovanadates for Multistate Resistive-Switching Memory Applications
F. Yang, M. Moors, D. A. Hoang, S. Schmitz, M. Rohdenburg, H. Knorke, A. Charvat, X. Wang, K. Yu. Monakhov, J. Warneke
ACS Appl. Nano Mater. 2022
https://doi.org/10.1021/acsanm.2c03025If someone wants to use negative molecular ions as single-molecule switching and storage units, an intrinsic problem arises: ions usually deposit with their counterions from solution onto surfaces, forming larger aggregates (quasi small salt crumbs). By mass selection of the anions and soft-landing them in vacuum, these counterions can be excluded and the deposition of single molecular storage units is thereby possible. Initially, the method was not very successful because the switchable anions were simply not stable on surfaces without counterions and decomposed under ambient condition. To counteract this, researchers used a trick from so-called host-guest chemistry. A circular sugar molecule wraps itself around the negative ion as a "ring" and protects it. Thus, the molecular storage unit remains stable even on surfaces and can be successfully switched through several redox states. This opens up new avenues for the preparation and addressing of memory units with a control of the adsorption properties at the single molecule level.
