The generation of novel metal oxide materials for semiconductor technology is of crucial importance for the improvement of the efficiency of electronic components and the associated energy savings. These metal oxides are aimed to enable access to more powerful micro- and nanoelectronics for “Green IT”. However, the preparation of complex metal oxide materials (defined as metal oxide materials with several metals, phases or additional dopants) can be limited by classical production methods. Their development usually requires high-temperature solid-state chemistry synthesis, where a high degree of homogeneity of the various metals and dopants in the product is difficult to achieve. An alternative way is the exploitation of multimetallic molecular precursor materials, with the help of which the preparation of homogeneous complex metal oxides down to the atomic level and at much lower temperatures is potentially possible.
The synthesis of such air, humidity and temperature stable molecules (on the scale of metalorganic compounds), which comprise at least two types of metals, can be handled well under ambient conditions. The obtained products are characterized by powder and single crystal X-ray diffraction (XRD), thermogravimetric analysis (TGA), infrared spectroscopy (IR) and cyclic voltammetry (CV).
Next, the pre-selected molecules are deposited on solid substrates by e.g. spin- and dip-coating or drop-casting. The adsorption properties of the molecules can be optimized through controlled derivatization of the organic ligand shell of the respective metal-oxo core. The sub-monolayers or multilayers generated in this way are examined with respect to their interfacial interactions and surface properties. The characterization is carried out by surface-sensitive methods such as ellipsometry, XPS and EDX as well as imaging methods such as scanning tunneling microscopy (STM) and atomic force microscopy (AFM), which provide insights into the structural and electronic properties of the molecules and their thin films.
These multimetallic-based molecular thin films can potentially be converted into new types of metal oxide thin films with yet unexplored electronic characteristics. Investigations of the conductivity and the switching of both the molecular and the new metal oxide thin films are the focus of studies in the subgroup “Molecular oxide switching materials”.
Highlights
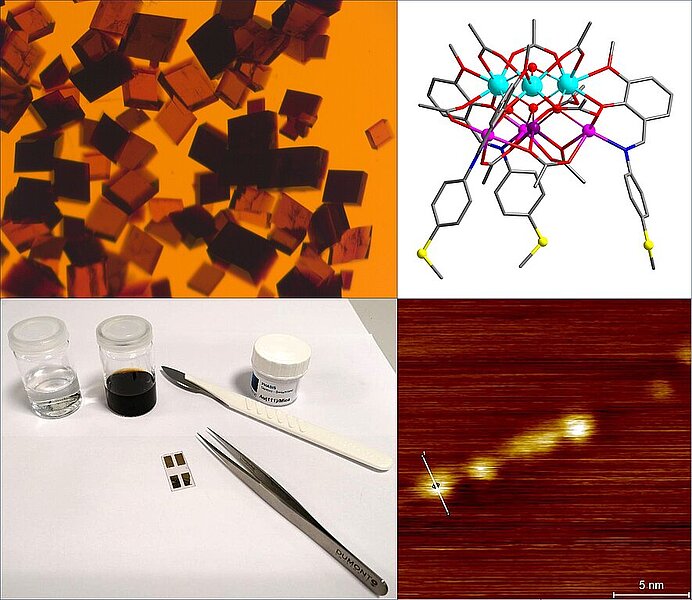
Synthesis, Structure, and Surface Adsorption Characteristics of a Polynuclear MnII,IV–YbIII Complex
K. Ueltzen, S. Schmitz, M. Moors, M. Glöß, M. Börner, I. Werner, Z. Warneke, J. Warneke, B. Abel, K. Y. Monakhov
Inorg. Chem. 2021, 60, 10415–10425
https://pubs.acs.org/doi/10.1021/acs.inorgchem.1c00994The controlled adsorption of polynuclear coordination compounds with specific structural and electronic characteristics on surfaces is crucial for the prospective implementation of molecule–surface interfaces into practical electronic devices. From this perspective, a neutral 3d,4f-coordination cluster [MnII3MnIVYb3O3(OH)(L·SMe)3(OOCMe)9]·2MeCN·3EtOH (1·2MeCN·3EtOH), where L·SMe– is a Schiff base, has been synthesized and fully characterized and its adsorption on two different solid substrates, gold and graphite, has been studied. The mixed-valence compound with a bilayered metal core structure and the structurally exposed thioether groups exhibits a substantially different surface bonding to metallic gold and semimetallic graphite substrates. While on graphite the adsorption takes place only on distinguished attraction points with a locally increased number of potential bonding sites such as terrace edges and other surface defects, on gold the molecules were found to adsorb rather weakly on randomly distributed adsorption sites of the surface terraces. This entirely different behavior provides important information for the development of advanced surface materials that may enable well-distributed ordered molecular assemblies.
Expansion of Zirconium Oxide Clusters by 3d/4f Ions
S. Schmitz, N. V. Izarova, J. van Leusen, K. Kleemann, K. Y. Monakhov, P. Kögerler
Inorg. Chem. 2021, 60, 11599–11608
https://pubs.acs.org/doi/10.1021/acs.inorgchem.1c01526Two series of charge-neutral coordination clusters featuring quasi-isostructural metal oxide cores, isolated as [Zr6Fe2Ln2O8(ib)14(bda)2(NO3)2]·xMeCN (Ln = La (1), Ce (2), Pr (3), and Nd (4); ib– = isobutyrate; H2bda = N-butyldiethanolamine) and [Zr6Fe2Ln2O8(ib)14(mda)2(NO3)2]·xMeCN (Ln = La (5), Ce (6), Pr (7), and Nd (8); H2mda = N-methyldiethanolamine), were obtained via one-pot reactions of [Fe3O(ib)6(H2O)3]NO3 as a critical precursor, Ln(NO3)3·6H2O (Ln = La, Ce, Pr, and Nd), the respective aminoalcohol, and [Zr6O4(OH)4(ib)12(H2O)]·3Hib in an acetonitrile solution. The coordination clusters in 1–8 feature {Zr6O8} cores that are structurally expanded by two 4f (Ln3+) and two 3d (Fe3+) metal ions, each individually coordinated to one of the eight oxide centers of {Zr6O8}, producing a metal skeleton where the 3d/4f positions cap four of the triangular faces of the central Zr6 octahedron. The coordination clusters differ in the chosen aminoalcohol coligands, N-butyldiethanolamine or N-methyldiethanolamine, which lead to a different isobutyrate coordination pattern in the two series, while the {Fe2Ln2Zr6O8} core structure remains virtually unaffected. All eight coordination clusters are obtained in moderate to good yields of 29–66% after only several days. Complexes 1–8 are stable against air and moisture; they are also surprisingly thermally stable up to 280 °C in air and in nitrogen atmosphere, and they represent the first reported examples of 3d/4f-functionalized zirconium oxide clusters.
Conductive Self-Assembled Monolayers of Paramagnetic {CoIICoIII4} and {CoII4CoIII2} Coordination Clusters on Gold Surfaces
S. Schmitz, X. Qiu, M. Glöß, J. van Leusen, N. V. Izarova, M. A. Nadeem, J. Griebel, R. C. Chiechi, P. Kögerler, K. Y. Monakhov
Front. Chem. 2019, 7, 681
https://www.frontiersin.org/articles/10.3389/fchem.2019.00681/fullTwo polynuclear cobalt(II,III) complexes, [Co5(N3)4(N-n-bda)4(bza·SMe)2] (1) and [Co6(N3)4(N-n-bda)2(bza·SMe)5(MeOH)4]Cl (2), where Hbza·SMe = 4-(methylthio)benzoic acid and N-n-H2bda = N-n-butyldiethanolamine, were synthesized and fully characterized by various techniques. Compound 1 exhibits an unusual, approximately C2-symmetric {CoIICoIII4} core of two isosceles Co3 triangles with perpendicularly oriented planes, sharing a central, high-spin CoII ion residing in a distorted tetrahedral coordination environment. This central CoII ion is connected to four outer, octahedrally coordinated low-spin CoIII ions via oxo bridges. Compound 2 comprises a semi-circular {CoII4CoIII2} motif of four non-interacting high-spin CoII and two low-spin CoIII centers in octahedral coordination environments. Self-assembled monolayers (SAMs) of 1 and 2 were physisorbed on template-stripped gold surfaces contacted by an eutectic gallium-indium (EGaIn) tip. The acquired current density-voltage (I-V) data revealed that the cobalt-based SAMs are more electrically robust than those of the previously reported dinuclear {CuIILnIII} complexes with Ln = Gd, Tb, Dy, or Y (Schmitz et al., 2018a). In addition, between 170 and 220°C, the neutral, mixed-valence compound 1 undergoes a redox modification, yielding a {Co5}-based coordination cluster (1-A) with five non-interacting, high-spin octahedral CoII centers as indicated by SQUID magnetometry analysis in combination with X-ray photoelectron spectroscopy and infrared spectroscopy. Solvothermal treatment of 1 results in a high-nuclearity coordination cluster, [Co10(N3)2(N-n-bda)6(bza·SMe)6] (3), containing 10 virtually non-interacting high-spin CoII centers.